How to Avoid Clinical Investigation for Your Medical Device
Introduction
Under the Medical Device Regulation (MDR), clinical investigations are generally required for Class III and IIb implantable medical devices. However, specific exemptions allow manufacturers to avoid clinical investigation for medical devices while still demonstrating compliance with safety and performance requirements.
Understanding these exemptions, as outlined in MDR Article 61 and guidance documents such as MDCG 2023-7, MDCG 2020-5, and MDCG 2020-6, can help streamline regulatory approval and reduce unnecessary clinical study costs.
Case 1: Modification of a Marketed Device by the Same Manufacturer
If the Device Under Evaluation (DUE) is a modified version of an already marketed device from the same manufacturer, a new clinical investigation may not be required.
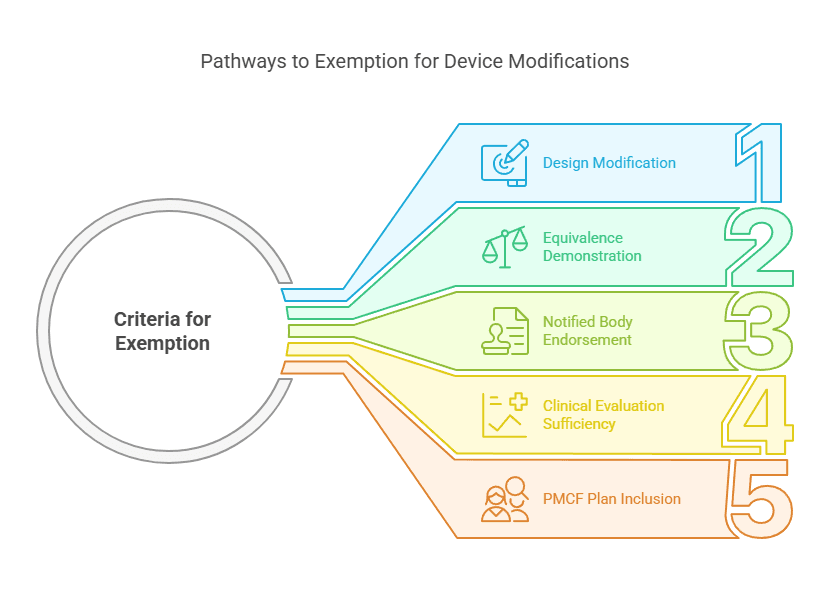
Criteria for Exemption:
The DUE is a design modification of an existing device from the same manufacturer.
Equivalence with the marketed device is demonstrated per Annex XIV Section 3.
The notified body endorses the equivalence claim.
The clinical evaluation of the marketed device is sufficient to demonstrate conformity with General Safety and Performance Requirements (GSPRs).
A Post-Market Clinical Follow-Up (PMCF) plan includes post-market studies to verify safety and performance.
Reference:
MDR Article 61(4), Indents 1-3
Case 2: Legacy Devices Placed on the Market Under Previous Directives
Devices that were legally placed on the market under Directive 90/385/EEC or Directive 93/42/EEC before the MDR came into effect may be exempt from clinical investigations if sufficient clinical data is available.
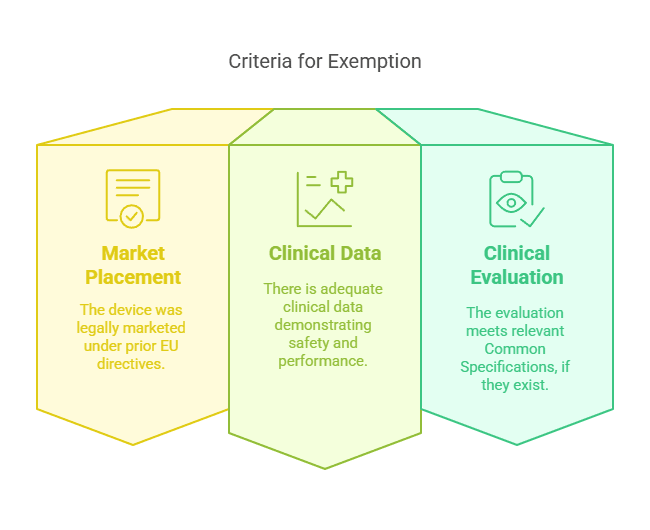
Criteria for Exemption:
The DUE was lawfully placed on the market under previous EU directives.
Sufficient clinical data supports the safety and performance of the device.
The clinical evaluation aligns with applicable Common Specifications (CS), if available.
Reference:
MDR Article 61(6)(a)
Well-Established Technology (WET) devices, such as sutures, staples, screws, and dental fillings, may avoid clinical investigation for medical devices if existing clinical data is sufficient.
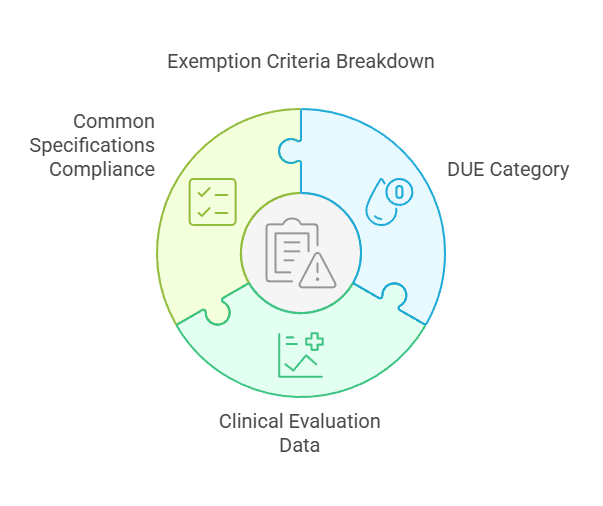
Criteria for Exemption:
The DUE falls within the category of WET.
The clinical evaluation is supported by sufficient clinical data.
Compliance with relevant Common Specifications is ensured, if applicable.
Reference:
MDR Article 61(6)(b)
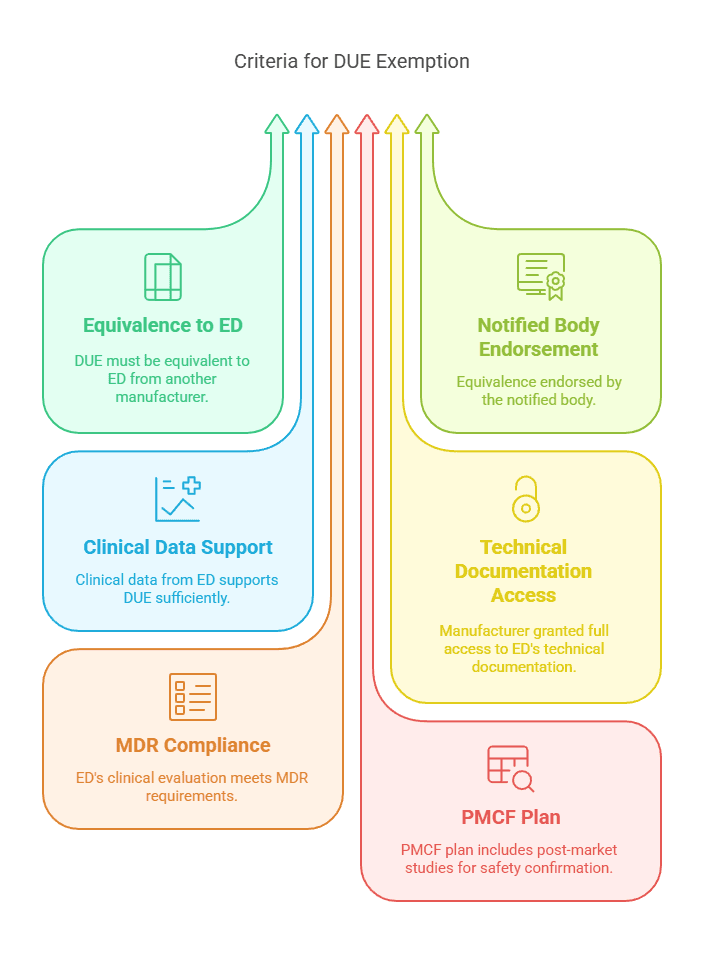
Case 4: Equivalent Device from Another Manufacturer
A manufacturer may avoid clinical investigation for a medical device by demonstrating equivalence to another manufacturer’s device, provided that full access to the technical documentation of the equivalent device (ED) is ensured through a contractual agreement.
Criteria for Exemption:
The DUE is equivalent to an ED from another manufacturer per Annex XIV Section 3.
Equivalence is endorsed by the notified body.
Clinical data from the ED sufficiently supports the DUE.
A contract explicitly grants the manufacturer full access to the ED’s technical documentation.
The ED’s clinical evaluation meets MDR requirements.
A PMCF plan includes post-market studies to confirm safety and performance.
Reference:
MDR Article 61(5)
Demonstration of Sufficient Access to Data
Annex XIV Section 3 of the MDR requires manufacturers to justify their level of access to clinical, technical, and biological data when claiming equivalence. However, a contract is only mandatory under Article 61(5).
Key Considerations:
Access refers to having enough data to support equivalence claims.
Full access to the ED’s technical file is only required under Article 61(5).
In other exemption cases, access can be justified through comparative analysis, publicly available information, or internal clinical data.
What About Other Device Classes?
For devices that are not Class III or implantable, proving equivalence does not require a formal contract. Instead, manufacturers must demonstrate appropriate access to sufficient clinical, technical, and biological data as per Annex XIV Section 3.
Key Considerations:
A contract is not required to prove equivalence for non-Class III or non-implantable devices.
Manufacturers must demonstrate access to sufficient clinical, technical, and biological data.
If the necessary conditions for equivalence are not met, a clinical investigation may still be required.
The contract requirement under Article 61(5) applies only to specific situations for Class III and implantable devices.
Conclusion
Manufacturers seeking to avoid clinical investigation for medical devices must carefully evaluate whether their device qualifies for one of the four exemptions under MDR Article 61. By strategically leveraging existing data, demonstrating equivalence, and implementing robust PMCF plans, companies can optimize regulatory compliance without unnecessary clinical studies.
Final Takeaways:
MDR Article 61 provides specific exemptions to avoid clinical investigation for medical devices.
Properly documented equivalence and sufficient clinical data are essential.
Consulting with regulatory experts ensures compliance with exemption criteria.
Stay Updated with Exclusive Clinical Insights
For more expert insights on clinical evaluation strategies, MDR compliance, and regulatory pathways, subscribe to my newsletter. Get exclusive, in-depth guidance to streamline your clinical evaluation process and stay ahead in the medical device industry: Subscribe Here