Clinical Evaluation vs Clinical Investigation
When doing through the regulatory pathways for medical devices in the EU, healthcare innovators often encounter two fundamental concepts: clinical evaluation and clinical investigation. While they may seem similar at first glance, they serve distinct and crucial roles in ensuring device safety and performance.
What Is Clinical Evaluation?
In accordance with the European Union Medical Device Regulation (EU MDR), clinical evaluation is defined as a systematic and ongoing process. This process involves gathering, analyzing, and assessing clinical data to verify the device’s intended performance and safety, including clinical benefits.
But what does that really mean?
It means a manufacturer must continuously prove that a medical device does what it’s supposed to do without introducing undue risk to patients. Clinical evaluation draws from various data sources, such as:
Published scientific literature
Clinical investigations (if available)
Post-market surveillance (PMS)
Complaint trends and field safety notices
Rather than a one-time event, clinical evaluation is a living document. It’s regularly updated, especially after new findings arise in the post-market phase.
What Is a Clinical Investigation?
A clinical investigation, on the other hand, is a targeted study conducted on human subjects to generate new clinical data. Think of it as the scientific trial arm of device assessment.
The EU MDR defines it as a systematic investigation, typically carried out when existing clinical evidence is insufficient. This applies particularly to:
Innovative devices
Modified existing devices
Devices entering new indications
Additionally, the ISO 14155:2020 standard echoes the same definition, stressing the importance of such investigations in understanding clinical performance, effectiveness, and safety under real-world or controlled settings.
Why the Distinction Matters
Here’s where things get critical from a regulatory and practical standpoint. Understanding when to rely on clinical evaluation versus when to initiate a clinical investigation can significantly streamline your CE marking journey. Misjudging this can lead to:
Regulatory delays
Insufficient data for conformity assessment
Increased risk of non-compliance during audits
So, how do these two pathways differ exactly? Let’s break it down.
Purpose
Clinical Evaluation: Aims to continuously verify a device’s safety and effectiveness using existing data.
Clinical Investigation: Conducted to collect new clinical data, especially when existing data can’t support safety and performance claims.
Scope of Application
Clinical Evaluation: Covers a wide array of sources – prior investigations, scientific literature, PMS data…
Clinical Investigation: Focuses only on specific studies conducted under controlled conditions.
Regulatory Requirements
Clinical Evaluation: Mandatory for all medical devices, regardless of risk class. It’s a core part of the conformity assessment for CE marking.
Clinical Investigation: Required only when existing data is lacking or inadequate. For instance, novel implants or devices in Class III often fall into this category.
Lifecycle Phase
Clinical Evaluation: Conducted throughout the lifecycle of a device – from design to post-market.
Clinical Investigation: Usually occurs pre-market, but may also be conducted post-market under specific surveillance needs.
Real-World Example
Imagine a company developing a new orthopedic implant. While they’ve leveraged existing literature and PMS data from similar devices, the implant features a novel polymer.
Despite the existing data being somewhat supportive, regulatory experts would recommend a clinical investigation. Why? Because the material change introduces unknown variables – potential risks that must be studied in a clinical setting.
Once the investigation concludes, the clinical evaluation process resumes using both new and existing data to paint a holistic safety and performance profile.
Harmonizing Both Approaches
It’s important to note that clinical investigations feed directly into clinical evaluations. Data obtained from investigations becomes part of the Clinical Evaluation Report (CER).
Thus, rather than being isolated, the two are interdependent:
Investigations inform evaluations.
Evaluations highlight gaps that require investigations.
This feedback loop ensures the regulatory robustness of a device’s dossier, especially under scrutiny from Notified Bodies.
FAQs
What is the primary goal of clinical evaluation in medical devices?
To verify the safety, performance, and clinical benefits of a device using existing clinical data, ensuring it works as intended without posing risks.
When is a clinical investigation necessary under EU MDR?
When the available clinical evidence is insufficient to prove safety or performance—often with new, innovative, or significantly modified devices.
Can clinical evaluation use data from other devices?
Yes, provided those devices are equivalent in terms of design, composition, and intended purpose—and that equivalence is clearly justified.
Is a clinical investigation always required for Class III devices?
Not always, but it’s more common. The need depends on existing data availability, novelty of the device, and risk profile.
How often should the Clinical Evaluation Report be updated?
It should be updated regularly, especially after significant PMS findings, new literature publications, or major changes to the device.
Can post-market clinical follow-up (PMCF) be part of clinical investigation?
Yes. PMCF studies can be designed as clinical investigations when they aim to collect new long-term data.
Final Thoughts
If you’re a regulatory affairs specialist, quality manager, or startup innovator going through the EU MDR , here’s what you need to remember:
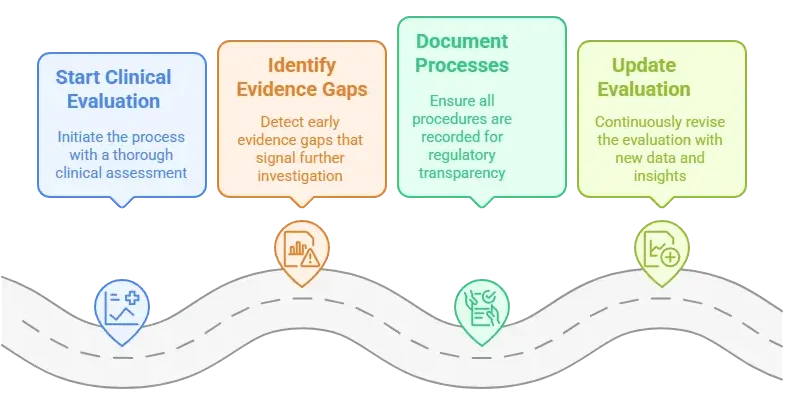
Always start with a clinical evaluation.
Identify evidence gaps early – that’s your red flag for a clinical investigation.
Document both processes meticulously – because regulators will expect transparent traceability.
Keep your evaluation up-to-date. That means integrating any new clinical data, PMS insights, or safety alerts.
By understanding the interplay between these processes, you ensure a safer, faster route to market – and more importantly, you protect patient health.
PS: For more information, subscribe to my newsletter and get access to exclusive content, private insights, and expert guidance on MDR compliance and CE marking: Subscribe Here
✌️ Peace,
Hatem Rabeh, MD, MSc Ing
Your Clinical Evaluation Expert & Partner
Follow me for more insights and practical advice!